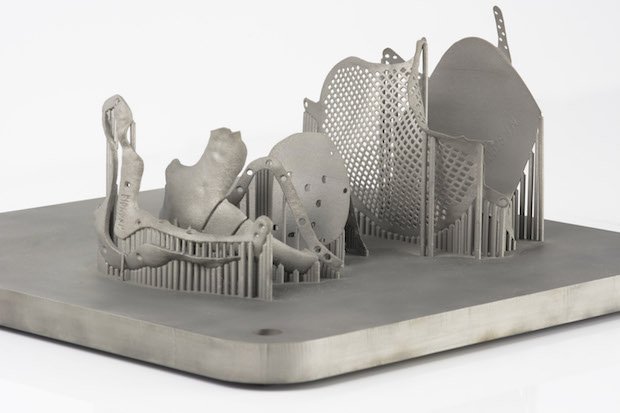
Additively manufactured medical devices are increasingly being used in a number of applications including dental frameworks, spinal and craniomaxillofacial (CMF) implants and orthopaedics. When manufacturing medical devices, additive manufacturing (AM) offers the ability to produce parts with complex geometry, specific to a patient’s anatomy.
Here, Dave Beeby, Principal Development Engineer, Additive Manufacturing Technology and Concepts at Renishaw, discusses the considerations for additively manufactured implants.
Additive manufacturing has been used to produce medical devices for more than ten years and the number of companies taking advantage of the technology for producing custom parts is on the rise. The technology allows implants to be produced out of high value materials like titanium, at a much lower cost than using traditional methods due to its additive nature. The medical technology industry is a natural fit for AM as it moves towards customisation, looks to improve efficiency and streamline production and delivery.
As well as allowing custom parts to be produced cost effectively, businesses can use AM technology to reduce time to market. What used to take weeks to manufacture or prototype using traditional methods can now be done in several hours. Advances in available software further reduces this by offering quick, easy to navigate design tools that companies or surgeons can use to generate device designs efficiently.
To take advantage of the benefits AM offers to implant manufacturers, companies must consider a variety of factors including; the regulation surrounding medical devices, material choice, software and machine optimisation.
One area of implant technology that has seen significant advancement in the last year is craniomaxillofacial surgery. This includes cranial plates, orbital floors, orbital walls, mid-face implants and mandibular bars and plates. AM technology can also be used to produce custom guides, which a surgeon can use to ensure an implant is correctly located.
This area of medicine is particularly well suited to AM technology, partly due to the variation in size and shape between patients, but also due to the benefits of customised guides to surgeons during procedures. Because of the benefits of AM technologies to surgeons, patients and hospitals in this field, significant research has been done to improve available software.
Software
CMF implant manufacture is traditionally based on physical models made of a patient’s head in plaster. For example, when producing a cranial plate, implants would be produced from a sheet of titanium. This sheet would be cut, wrapped around the model of the patient and pressed with a hydraulic press to form an implant capable of holding its shape. This process could take up to several weeks.
After the implant has been formed, it is then finished, deburred, cleaned, polished and necessary holes are drilled. Even after the completion of this intensive process, it is still possible that the shape may require additional manipulation during surgery to fit correctly.
Digitising the process can dramatically reduce the amount of time needed to produce an implant. The AM design process does not rely on physical models as the implant is designed in computer-aided-design (CAD) software, based on data from 3D patient scans.
Once it has been designed in CAD, the design is exported in a 3D mesh format, such as .stl, and then transferred into build preparation software to be prepared for the manufacturing process.
Designing for AM
Additive manufacturing removes many of the geometric constraints of traditional machining, but does come with its own considerations. It is important that the implant design is feasible for additive manufacturing. This means that it must be possible to add the correct supports to prevent thermal stress warping the part and to ensure the part is buildable.
Tailored software applications, such as ADEPT, a package for CMF implants, makes this easier. The software only allows the design of implants that can be manufactured, as it is programmed to take the design constraints of the process into account. The software means that the designer does not need to check for faults, giving the surgeon peace of mind that the implant is correct.
Implant manufacturers can use QuantAM build preparation software to carefully control the laser parameters of the AM machine. This is ideal for medical applications, as the machine parameters must be validated.
Surgeons and prosthetic teams can design implants for additive manufacture in the hospital. However, implant designers can work with third party manufacturers to benefit from their expertise and experience including their processes and quality management system. The hospital that commissioned the implant can use the already validated processes of its partner company and rely on its ISO13485 certification.
Structure
During the AM process, layers of powders are joined together to form a component using a laser. Without as many spatial limitations as traditional methods like CNC machining, implants can be designed with a form to fit the necessary function for the patient, rather than to fit in with the manufacturing constraints of the production process.
Another consideration when manufacturing custom implants is material choice and composition. The manufacturer must be sure that the resulting part has the necessary mechanical properties to meet medical device metallurgy standards. This includes achieving the lowest possible oxygen concentration and a high level of ductility to prevent the possibility of an implant snapping.
The manufacturer must also overcome any technical hurdles during process developments. With large, thin implants, there is a risk of warping. To achieve high accuracy and prevent warping, the manufacturer can develop a support strategy and optimise laser parameters to get the most accurate part.
However, significant process development is necessary to ensure that the product is suitable and meets regulation. By working with an experienced implant manufacturer, surgeons and implant designers can benefit from the manufacturer’s expertise.
For example, at Renishaw, we have developed processes to manufacture and finish each different CMF implant type, whether it is an orbital plate, mandible bar or cranial plate. This means that even though each implant has custom geometry, the part is produced according to a validated and robust process.
Developing an additive manufacturing implant process from scratch requires intensive research and development. By partnering with an experienced implant manufacturer, hospitals do not need to perform this work and can instead concentrate on their core skills, ultimately delivering greater benefit to the patient.
Bagikan Berita Ini














0 Response to "Design and manufacture considerations for medical implants"
Post a Comment